News - How do unregistered drugs appear on the market?
Business Strategy
How do unregistered drugs appear on the market?
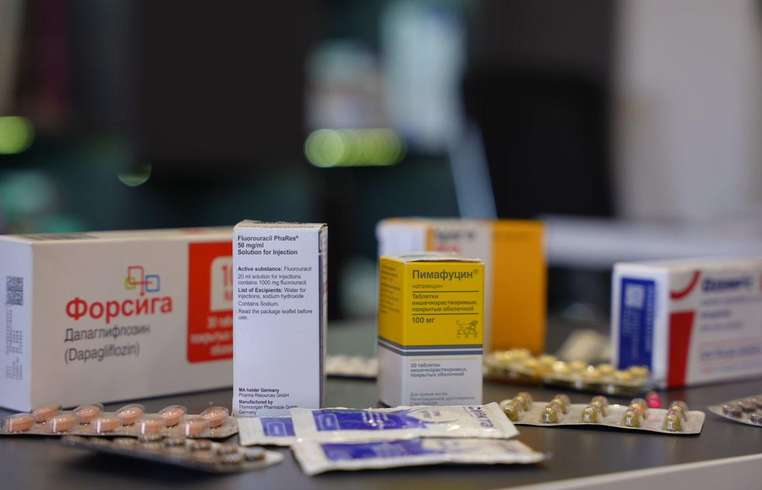
In Armenia, many medicines prescribed for cancer, diabetes, hormonal disorders and other illnesses are not registered, and therefore cannot be sold legally. Yet these medicines are freely sold in pharmacies or on the black market. Because they are not registered, no state body checks their effectiveness and safety. For years, doctors have warned that these medicines are life-saving for their patients and should be registered and properly tested to prevent counterfeit drugs from circulating, but the state has not managed to resolve the registration issue. Hetq obtained from one Yerevan pharmacy chain’s urban and regional branches 13 medicines that have no legal registration in Armenia. We are not naming the chain to avoid targeting a specific company, since it is possible that these medicines are sold in other chains as well. Why many important medicines are not registered in Armenia The drug registration process in Armenia is long and expensive. According to the Health Ministry, there are currently 3,661 drug names registered in Armenia. Marine Harutyunyan, head of the Drug Policy and Medical Technologies Department, says that some medicines do not reach Armenia not because of state restrictions but due to a lack of interest from manufacturers. Before registering a drug, it undergoes numerous studies, both documental and laboratory tests. If the results are positive, the drug is registered by a ministerial order for a five-year term. Harutyunyan notes that Armenia’s pharmaceutical market is small, which is why some manufacturers do not apply to register drugs. The registrant is either the drug owner or the manufacturer, meaning the necessary documents must be provided and owned by the manufacturer or authorized rights holders. Given the small market size, manufacturers’ interest often does not arise. According to Harutyunyan, rejections are not a matter of a minister or expert’s personal decision. Rejections are clearly defined by law and sub-legal acts. If there is a negative conclusion, such as unsatisfactory laboratory results or documentary deficiencies, and if these issues are not corrected within 180 days, registration is refused in line with legal requirements. Regarding drugs reaching Armenia through illegal channels, Harutyunyan says the problem stems from manufacturers’ interests. If a manufacturer does not request registration in our country, the state cannot compel them. But legislative changes have been introduced and there is a list of essential medicines. If a drug or dosage form is on that list, its import is allowed by ministerial order after documentary review. The black market for unregistered medicines is not under the Health Ministry’s control. The ministry does not have supervisory powers; oversight belongs to the State Health and Labour Inspectorate, which conducts checks almost daily. If an unregistered drug sale is recorded, severe penalties and fines are imposed. To import medicines, an organization must be licensed, have warehouses and qualified staff. During import, documents are reviewed by customs authorities and an expert center. The process takes 7–10 working days, after which a positive or negative conclusion is issued. Harutyunyan emphasizes that legally imported unregistered medicines are usually essential. They concern only legal imports. Procurement through state purchases is allowed not only for the health and defense ministries but also for medical institutions. However, all unregistered medicines must be manufactured and registered in the EEU member countries. Importing unregistered medicines from other countries is not allowed. Nevertheless, Armenian pharmacies sell medicines that are not produced by EEU member countries, nor have they reached Armenia through authorized channels with ministerial permission. Is the sale of unregistered medicines in Armenia being properly supervised? Harutyunyan responds that he cannot assess this because the function is not within the ministry’s remit. But there are clear legal and regulatory acts governing supervision, and as far as they know, supervision is carried out. How medicines are sold in pharmacies Hetq bought the following medicines: - Ozempic 0.5 mg (series PP5N094, expiry May 2027, purchased August 26, 2025). The drug was sold without a stamp label and with a fiscal receipt. - Tegretol 200 mg (series TLHW5, expiry January 2027, purchased July 24, 2025). Both the fiscal receipt and the stamp label were present. - Forsiga 10 mg (series 2720325, expiry February 2028, purchased August 1, 2025). The fiscal receipt and the stamp label were present. - Pimafucin 100 mg tablets (series 020290, expiry October 2027, purchased July 23, 2025). The fiscal receipt and the stamp label were present. - Baralgin (series 4P0563A, expiry January 2028, purchased July 23, 2025). The fiscal receipt and the stamp label were present. From the data on the drug box labels, it became evident that the importer entered data into the label registry that had nothing to do with the drug. Instead of the drug’s correct ATC/HS code (3004), an unpackaged vitamin code (2936) was entered. This analgesic and antipyretic product is also listed as a non-certified vitamin. - Omnic (series 571124, expiry July 2028, purchased July 23, 2025). The fiscal receipt is present, but the stamp label is missing. - Vesicar (series 154378, expiry April 2026, purchased July 23, 2025). It was sold without a fiscal receipt and without a stamp label. - Allohol (Pharmstandard, series 740524, expiry April 2028, purchased July 23, 2025). The fiscal receipt is present, but the stamp label is missing. - Smecta (series C56167, expiry April 2027, purchased July 23, 2025). The fiscal receipt is present, but the stamp label is missing. - Attarax (series 409451, expiry June 2029, purchased July 23, 2025). The fiscal receipt is present, but the stamp label is missing. - Senade (series 484007, expiry April 2027, purchased July 22, 2025). The fiscal receipt is present, but the stamp label is missing. 12–13. Xeloda (series 2408547, expiry June 2027) and Fluorouracil 50 mg/ml (PhaRes, Germany, series 440700, expiry June 2026) were purchased August 12, 2025. The fiscal receipt is present, but the stamp label is missing. Hetq sent the list of medicines it bought to the Ministry of Health and asked which of them are certified. The ministry replied that among the 13 unregistered medicines only Tegretol, the Italian version, had an import‑certificate issued. In the Pimafucin case, the ministry rejected the company’s application, but that does not prevent the sale. For the other 12 medicines, the ministry did not submit any application in its Authorization Documents system. Nevertheless, these medicines are available in the pharmacy chain we visited. Drug registration testing is conducted by the Ministry of Health’s Center for Drugs and Medical Technologies. According to the center’s deputy director, Naira Romanyova, medicines in Armenia are registered through general and simplified procedures, as well as in accordance with Eurasian Economic Union rules. The simplified procedure applies to medicines registered in member countries of government‑designated international professional organizations or to medicines that are pre‑qualified by EEU rules. The drug registration timeline depends on the procedure: national simplified registration takes 31 days, general registration up to 150 days, and EEU registration up to 180 working days. Pharmaceutical companies must plan their imports with these timelines in mind. Re‑registration is faster. How unregistered medicines bypass customs and appear on the market Hetq submitted the list of unregistered medicines and the pharmacy addresses to the State Revenue Committee, along with the fiscal receipts. Three of the medicines carried fiscal stamps, meaning their sale occurred within the legal tax framework despite not being registered. Twelve medicines came with receipts showing their drug names. We asked the SRC to explain: - The cases of violations of fiscal receipt rules, especially providing receipts without purchase documents; - The reasons for missing stamp labels on some medicines; - The legality of the presence or absence of stamp labels on certain medicines. The SRC replied that a thematic tax audit would be conducted and that results would be communicated, but no timeline was provided. High‑risk market, weak supervision Medicines circulation is monitored by the State Health and Labour Inspectorate, which operates under the Government. According to the inspectorate, in 2024 they checked 199 drug importers and sellers, issued 160 acts, and applied administrative liability in 838 cases. Nareк Karapetyan, head of the inspectorate’s Medicines Circulation Department, notes that unregistered or illegally imported medicines are detected, and orders to destroy the quantities are issued. If the operator does not comply, administrative liability follows. In addition to planned inspections, there is a control‑purchase mechanism. By government order, a control purchase is conducted under the directive of the inspectorate’s head, and once the directive is issued, the control purchase begins, followed by an inspection. When asked what grounds justify a control purchase — a complaint, a suspicion, or suspicious data — Karapetyan said that control purchases are performed only within the framework of inspections under the law. If an organization is on the annual inspection list, it may also be subject to control purchases. If information or a complaint reaches the inspectorate, administrative proceedings are opened, inspections are conducted, and, if necessary, the medicines are referred to the expert center to determine whether import and registration were conducted in accordance with the law. Pharmacies pay fines and continue selling unregistered medicines. If there is no complaint or alert, supervision may be repeated next year at best, if the company is included in the inspection list. In other words, even after penalties, a trader is not barred from continuing to sell an unregistered medicine. The actions of the Health Ministry, the Inspectorate and the State Revenue Committee show that oversight of medicines in the market is weak. The health system’s three main supervising bodies essentially do not cooperate closely. That gap allows unregistered medicines to be sold freely. Even after penalties, sales do not stop. Therefore, the state should find a way to register medicines deemed essential so that pharmacies do not violate rules and patients can use quality medicines.